Results from a recent study suggest that the mushroom species Coriolus versicolor (CV) may have therapeutic potential for the prevention and treatment of inflammatory bowel diseases. (1)
Using a mouse colitis model, the study found that CV biomass reduced histological signs of damage, attenuated the rise in multiple markers of inflammation, and boosted anti-inflammatory responses.
The study
Researchers from collaborating universities in Italy and the US induced colon damage in mice by intrarectally administering dinitrobenzenesulphonic acid (DNBS). Four groups of 10 mice were used: sham (intrarectal administration of 100 µL 50% ethanol); sham + Coriolus versicolor (200 mg/kg dissolved in saline, orally 1 h after sham procedure every 24 h); DNBS-treated (4 mg in 100 µL of 50% ethanol per mouse); and DNBS-treated + CV (200 mg/kg dissolved in saline, orally 1 h after DNBS procedure and every 24 h). Four days after the procedures, the mice were sacrificed.
Effects on physical appearance of the colon, weight loss and colon physiology
- Physical appearance
The colons of the sham groups appeared normal. Those of the untreated colitis model group were flaccid, with liquid stools and, in some cases, were ulcerated with mucosal congestion. These changes were significantly less in the CV-treated colitis model group. - Body weight
The significant loss of body weight (caused by diarrhea) in the colitis model group was significantly reduced in the CV-treated colitis model group. - Physiology
Histological analysis of sections of colon tissue revealed that DNBS had induced infiltration of inflammatory cells (mostly neutrophils – also see later), necrosis and oedema; although present in the CV-treated colitis model group, these pathological changes were significantly less marked.
Consuming Coriolus versicolor reduced the harm caused by DNBS, and better preserved the integrity of colon tissue.
Effects on inflammatory processes
The effects of CV in preventing inflammation of the colon were investigated by assessing multiple markers of increased inflammation.
- TLR4/NFĸB inflammatory pathway
Immunohistochemical analysis indicated that expression of the receptor TLR4 increased in the untreated colitis model mice, as did Myd88 expression, degradation of IĸB-α and nuclear translocation of NF-ĸB – all components involved in the TLR4/NFĸB inflammatory pathway. Together, these changes indicated upregulation of the inflammatory process. These pro-inflammatory effects were significantly limited in the CV-treated colitis model mice.
- Infiltration of neutrophils and lipid peroxidation
Neutrophil infiltration into the colon was assessed by measuring myeloperoxidase levels; the extent of lipid peroxidation in the colon was determined by activity of malondialdehyde. The large increases in these parameters (both of which suggest a pro-inflammatory status) in untreated colitis model mice were significantly attenuated in the CV-treated colitis model mice.
- Activation of T cells and adhesion molecules
The number of CD4+ and CD8+ (T cells that are important mediators of inflammation), and the expression of the adhesion molecules P-selectin (a transmembrane protein that has an essential role in inflammatory response to injury) and ICAM1 (a cell surface glycoprotein involved in recruiting active leucocytes to sites of inflammation) was assessed using immunohistochemical tissue staining. All parameters were significantly increased in the untreated colitis model mice, while their rise in CV-treated colitis model mice was significantly limited.
- Proinflammatory mediators
ELISA testing showed that proinflammatory mediators – CCL2, PGE2, IL-1β and TNF-α – were raised in the untreated colitis model mice, but significantly less-so in the CV-treated colitis model mice.
- Nitrosative stress and PARP hyperactivation
The levels of nitrosative stress and extent of PARP activity in colon samples – both indicative of potential for cell damage – were assessed immunohistochemically. There was a high level of positive staining of samples taken from colitis model mice (indicating a high potential for cell damage), with significantly less staining (and thus lower potential for damage) in samples from the CV-treated colitis model mice.
Consuming Coriolus versicolor significantly dampened the inflammatory processes, and the potential for cell damage, provoked by DNBS.
Enhancement of anti-inflammatory pathways
- Reactive oxygen species are important potentiators of inflammation; and Nrf2 is a protein responsible for controlling the expression of genes encoding enzymes that detoxify and eliminate reactive oxygen species (known as HO-1 enzymes). Western blot analysis of colon samples revealed that colitis model mice that had consumed CV had a much higher expression of Nrf2 and HO-1 than mice that had not consumed CV (both sham-operated and colitis model); this indicated that CV-treated mice were better able to ameliorate the effects of oxidative stress.
Consuming Coriolus versicolor enabled mice to better defend against the damaging effects of the oxidative stress induced by DNBS.
What do these findings mean?
As ASD is a spectrum disorder, many, but not all ASD patients suffer from gastrointestinal comorbidities, including acute and chronic constipation and diarrhoea as well as persistent neuroinflammation. Therefore, addressing this set of comorbidities is important as well as addressing neuroinflammation.
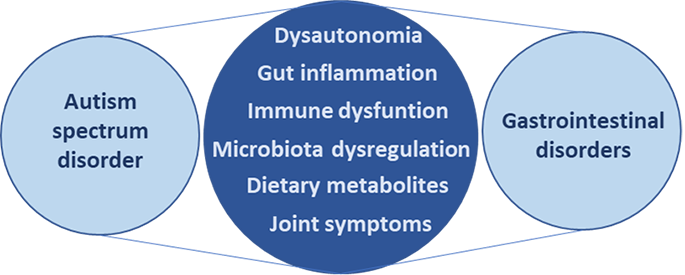
(Wasilewska J, Klukowski M. Gastrointestinal symptoms and autism spectrum disorder: links and risks – a possible new overlap syndrome. Pediatric health, medicine and therapeutics. 2015; 6: 153-166.)
In this colitis model study, consumption of Coriolus versicolor significantly reduced macro- and microscopic colon damage and the harmful inflammatory processes induced by DNBS in mice. It also heightened the mice’s protective anti-inflammatory response to DNBS challenge. These results suggest that Coriolus versicolor supplements hold great promise as a useful way to manage inflammatory bowel diseases.
Further studies are needed to fully understand the mechanisms behind Coriolus versicolor’s effects, and to confirm its potential as a disease modifying therapy in patients.
The Coriolus versicolor biomass used in the study was supplied by Mycology Research Laboratories Ltd (Luton, United Kingdom) and is a constituent in Hericor-MRL.
Reference
(1) Impellizzeri D, Fusco R, Genovese T, Cordaro M, D’Amico R, Trovato Salinaro A, Ontario ML, Modafferi S, Cuzzocrea S, Di Paola R, Calabrese V, Siracusa R. Coriolus Versicolor Downregulates TLR4/NF-ĸB Signaling Cascade in Dinitrobenzenesulfonic Acid-Treated Mice: A Possible Mechanism for the Anti-Colitis Effect. Antioxidants 2022;11:406. https://doi.org/10.3390/antiox11020406
Additional references with respect to Coriolus versicolor with respect to inflammation and neuroinflammation
D’Amico, R.; Trovato Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R.; et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants 2021, 10, 898. [CrossRef]
Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato Salinaro, A.; Maiolino, L.; Calabrese, V. (2020). Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2020, 21, 284. doi: 10.3390/ijms21010284
Bell V, Ferrão J, Chaquisse E, Manuel B and Fernandes T. Role of Mushrooms in Autism. Austin J Nutri Food Sci. 2019; 7(6): 1128.
Ferreiro E, Pita IR, Mota SI, Valer J, Ferreira NR, Fernandes T, Calabrese V, Fontes-Ribeiro CA, Pereira FC, Rego AC. (2018). Coriolus versicolor biomass increases dendritic arborization of newly-generated neurons in mouse hippocampal dentate gyrus. Oncotarget. 2018; 9(68):32929- 32942. doi:10.18632/oncotarget.25978. https://www.ncbi.nlm.nih.gov/ pubmed/30250640
Trovato A, Pennisi M, Crupi R, Di Paola R, Alario A, Modafferi S, Di Rosa G, Fernandes T, Signorile A, Maiolino L, Cuzzocrea S, Calabrese V. (2017). Neuroinflammation and mitochondrial dysfunction in the pathogenesis of Alzheimer’s Disease: modulation by Coriolus versicolor (Yun-Zhi) nutritional
Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [CrossRef]