The family of a two-year old boy with a rare epileptic condition are taking legal action to fight for potentially life-saving medication for him.
Charlie Hughes has been denied the medication because of a disagreement between his NHS Trust and the National Institute for Heath and Care Excellence (NICE).
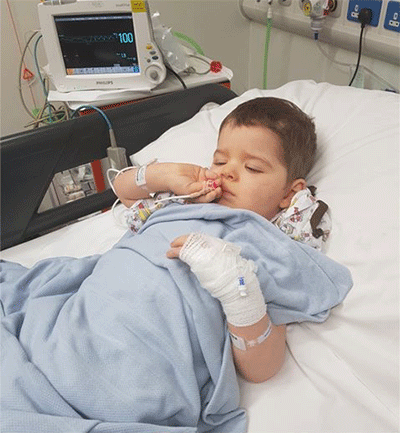
Charlie Hughes before his treatment began with medicinal cannabis
The disagreement is about the effect of guidelines that NICE has published on the use of cannabis-based medicinal products (CBMPs).
Refused legal aid
Charlie’s parents have been refused legal aid to challenge their NHS Trust. They are trying to raise money by crowdfunding to enable them to continue in the fight for Charlie’s wellbeing. Click here to visit the crowdfunding site.
Doctors diagnosed Charlie with Infantile Spasms at ten weeks old. Later, they diagnosed him with West Syndrome, a rare condition characterised by spasms and developmental delay.
He has in the past suffered up to 100 spasms a day and his condition has been largely resistant to anti-epileptic medication.
Damage to his developing brain
Doctors have confirmed that Charlie’s seizures are causing damage to his developing brain. Any treatment that reduces these seizures will reduce the damage they are causing.
Conversely, withdrawing any such treatment will likely result in further damage and could have life-threatening consequences.
However, in May 2019 Charlie started taking two CBMPs, Bedrolite and Bedica. His parents obtained them from a private doctor. The drugs are manufactured in Holland according to EU guidelines for good manufacturing practice.
After starting to take the drugs, Charlie’s seizures reduced to less than ten a day. Some days he was seizure free.
There was also a significant improvement in his social and physical development. Charlie was happier, more alert, far more vocal and constantly babbling, and he began taking an interest in toys.
Significantly reduced epileptic activity
The family says an electroencephalogram (EEG) in October 2019 provided objective evidence that the drugs were working. The EEG showed showing significantly reduced epileptic activity in Charlie’s brain.
The family reports that Charlie’s NHS doctors accept there has been a significant improvement in Charlie’s condition due to the cannabis-based products. The doctors said they would prescribe them if they could.
However, the NHS Trust responsible for Charlie’s care says the NICE Guideline on the prescription of medicinal cannabis prevents them from prescribing it.
NICE, on the other hand, says its Guideline does not prevent doctors making prescriptions where it is clinically appropriate. But the Guideline’s wording does not give doctors and NHS Trusts the confidence they need to prescribe the products.
Still refused a prescription
Charlie’s MP, Chloe Smith, wrote to Matt Hancock, the Secretary of State for Health and Social Care, to ask for a resolution of the situation. Mr Hancock responded that he was “determined that patients should receive [cannabis-based] products where it is clinically appropriate”.
But the situation has not changed for Charlie. His NHS Trust is still refusing to write a prescription.
As a result, Charlie’s parents have to pay thousands of pounds a month to buy the drugs privately. This is not affordable in the long term and they worry that they will run out of money, with life-threatening consequences for Charlie.
They feel they have little option but to bring legal proceedings in the form of a judicial review against both NICE and the NHS Trust.
Damning ramifications
Alice Hardy, a partner at Hodge Jones & Allen, is representing Charlie’s family. She said: “Charlie’s family are challenging the failure of the NHS to prescribe what, for their child, is a life-transforming, and possibly lifesaving, treatment. The failure of NICE to take a clear position on whether CBMPs can be prescribed in such cases has had damning ramifications for Charlie’s family and so many others in the same position.”
Alison Hughes, Charlie’s mum, said: “Charlie is only two years old, but he has spent the whole of his life trying different medications, many unlicensed for children and most with damaging effects on the developing brain.
“Finally, we’ve arrived at a treatment that is helping his condition. We are desperate to have CBMPs approved. Every day that we don’t see justice is another day of suffering for Charlie.”
The challenge is part of a wider campaign on behalf of other families affected by treatment-resistant epilepsy. These other families have also seen benefits from medical cannabis but are unable to access it on the NHS.
The campaigning group End Our Pain aims to secure legislation for access to whole-plant medical cannabis for these families. It hopes this challenge will result in positive changes for them, too.
Related:
- Hopes dashed over medical cannabis
- Cannabis trial results ‘exciting’
- Cannabis tested on men with autism
- Urgent review call for cannabis medicine
- US Army tests cannabis extract on autism
- World-first trial of cannabis for autism
- Drugs agency halts cannabis treatments
Published: 23 February 2020